Improved Mobility.
Made Easier.TM
The L300 Go system uses functional electrical stimulation (FES) to help correct foot drop and thigh weakness in people coping with the effects of stroke, multiple sclerosis, and other medical conditions.
A Superior Standard of Care
Research has demonstrated the ability of functional electrical stimulation to improve clinical outcomes.

Reduces Fall Risk

Prevents Muscle Atrophy

Promotes Neuroplasticity

Improves Range of Motion

Improves Critical Balance Measures

Increases Confidence and Independence
L300 Go promotes recovery by activating the neuromuscular pathways required for gait.

Walk More Naturally
L300 Go is a functional electrical stimulation (FES) system capable of producing measurable mobility improvements for patients with foot drop and/or knee instability caused by an upper motor neuron disease or injury such as:
- Stroke
- Multiple Sclerosis
- Cerebral Palsy
- Traumatic Brain Injury
- Incomplete Spinal Cord Injury
What is the L300 Go System?
The L300 Go is the world’s first FES system that integrates proprietary 3D motion detection. Through an adaptive, learning algorithm, the L300 Go detects gait events, providing stimulation precisely when needed making it easier to clear their foot at varying walking speeds, on stairs, ramps, and while navigating uneven territory.
45+ Research Articles and Level 1A Evidence
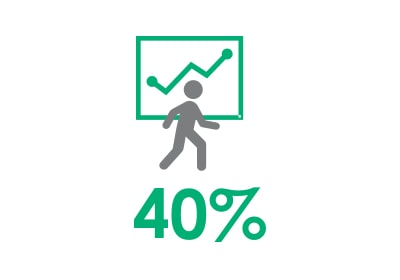
40% improvement in walking speed3

15% improvement on critical balance measures4 (decreased fall risk)
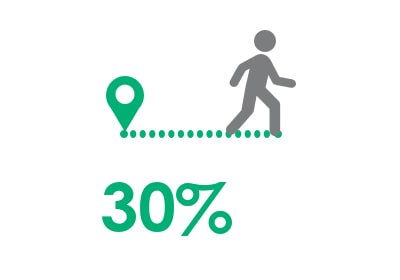
30% improvement in distance walked5
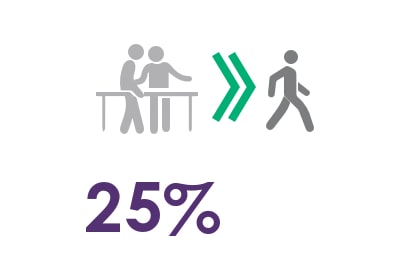
25% therapeutic improvement6
L300 Go. More Capable and Versatile.

3D Motion Detection

Fast, Intuitive Set-Up

Mobile App
Benefits Comparison
L300 Go vs. Ankle Foot Orthosis (AFO)
| Benefit | L300 Go | AFO |
|---|---|---|
| Neuroplasticity promotion | Yes | No |
| Carry-over effect when not wearing device | Yes | No |
| Improved range of motion | Yes | No |
| Improved critical balance measures | Yes | No |
| Muscle atrophy prevention | Yes | No |
| Superior patient satisfaction | Yes | No |
L300 Go Configurations

Lower Cuff for Foot Drop

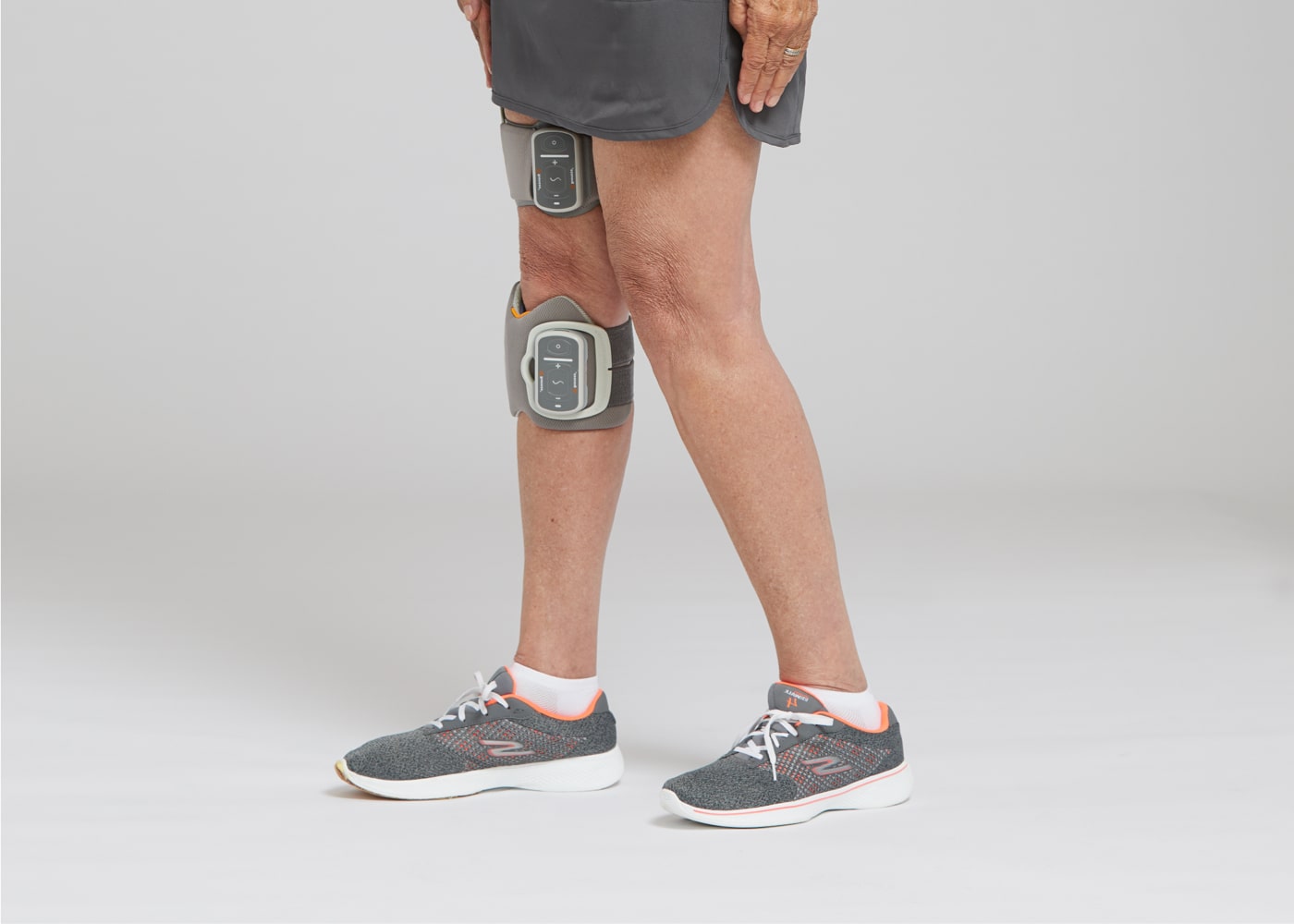
Lower Cuff and Thigh Plus for Foot Drop and Knee Instability

Small Lower Cuff for Pediatric Foot Drop

Thigh Stand-Alone for Knee Instability and Thigh Weakness
Contact us to schedule a demo with L300 Go or if you have questions about other Bioness products.
Indications for Use: The L300 Go System is intended to provide ankle dorsiflexion in adult and pediatric individuals with foot drop and/or to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L300 Go System electrically stimulates muscles in the affected leg to provide ankle dorsiflexion of the foot and/or knee flexion or extension; thus, it also may improve the individual’s gait.
The L300 Go System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, and increase local blood flow.
L300 Go is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l300/safety-information.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 800-211-9136 to request an electronic copy.

